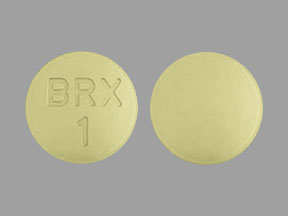
Otsuka and Lundbeck Issue Statement on U.S. Food and Drug Administration (FDA) Advisory Committee Meeting on REXULTI® (brexpiprazole) for the Treatment of Agitation Associated with Alzheimer's Dementia
Por um escritor misterioso
Last updated 18 maio 2024

Otsuka Pharmaceutical Development & Commercialization, Inc., (Otsuka) and Lundbeck Pharmaceuticals LLC (Lundbeck) announce the Joint Meeting of th
FDA Approves First-Ever Drug for Agitation Associated with
Otsuka Files a Supplemental Application in Japan: A Plastic Case
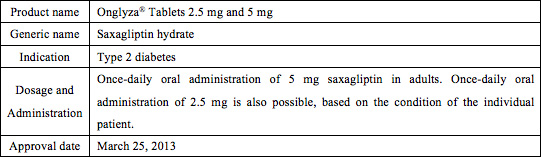
Otsuka Obtains Marketing Approval in Japan for Onglyza®

Piyush Dham on LinkedIn: The caring company: At Otsuka America

Otsuka Europe Development and Commercialisation Ltd., a New

Brian Ruhl on LinkedIn: The Neurological Benefits of Building Trust

Otsuka Supports Light-Up in Green Glaucoma Awareness Activities

Lundbeck on LinkedIn: Otsuka and Lundbeck Issue Statement on U.S.

Otsuka And Lundbeck Issue Statement On Food And Drug, 59% OFF

Gate Neurosciences opens lab in NorthShore Evanston Hospital
Otsuka Pharmaceutical and Shanghai Rightongene
/cloudfront-us-east-2.images.arcpublishing.com/reuters/4UHG5G2BNFNIBNXZS6VPJ7GDZ4.jpg)
Japan's Otsuka Pharma gets FDA approval for Alzheimer's agitation
Recomendado para você
-
 REXULTI® (brexpiprazole) Patient Information Website18 maio 2024
REXULTI® (brexpiprazole) Patient Information Website18 maio 2024 -
 Order Rexulti 2 mg with free shipping - Online Canadian Pharmacy18 maio 2024
Order Rexulti 2 mg with free shipping - Online Canadian Pharmacy18 maio 2024 -
 Rexulti (Brexpiprazole): Side Effects, Use for Depression, and More18 maio 2024
Rexulti (Brexpiprazole): Side Effects, Use for Depression, and More18 maio 2024 -
 Rexulti vs Abilify: Which is best for you?18 maio 2024
Rexulti vs Abilify: Which is best for you?18 maio 2024 -
 REXULTI TV Spot, 'Isolated'18 maio 2024
REXULTI TV Spot, 'Isolated'18 maio 2024 -
 FDA chides Otsuka for making false or misleading claims in Rexulti advertising18 maio 2024
FDA chides Otsuka for making false or misleading claims in Rexulti advertising18 maio 2024 -
 Rexulti 0,5mg 30 Comprimidos18 maio 2024
Rexulti 0,5mg 30 Comprimidos18 maio 2024 -
Rexulti: Uses, Side Effects & Warnings18 maio 2024
-
 REXULTI 3 MG (28 Compr. Recubierto) - Compra Online - Liga Chilena contra la Epilepsia18 maio 2024
REXULTI 3 MG (28 Compr. Recubierto) - Compra Online - Liga Chilena contra la Epilepsia18 maio 2024 -
 Rexulti For Depression: Benefits, Side Effects & Precautions18 maio 2024
Rexulti For Depression: Benefits, Side Effects & Precautions18 maio 2024
você pode gostar
-
 Has My Next Life as a Villainess season 3 been confirmed?18 maio 2024
Has My Next Life as a Villainess season 3 been confirmed?18 maio 2024 -
 Does Anyone Like The Alola Anime Art Style?18 maio 2024
Does Anyone Like The Alola Anime Art Style?18 maio 2024 -
 Protetor Motor Stunt Cage Mt 03 Mt03 Stunt Race Preto Fosco18 maio 2024
Protetor Motor Stunt Cage Mt 03 Mt03 Stunt Race Preto Fosco18 maio 2024 -
 Roblox PS4/PS5: How to Enable Quick-Login With Code Tutorial!18 maio 2024
Roblox PS4/PS5: How to Enable Quick-Login With Code Tutorial!18 maio 2024 -
Assistir The Café Terrace and Its Goddesses - online18 maio 2024
-
 Conheça os jogos para Educação Infantil que são alinhados à BNCC!18 maio 2024
Conheça os jogos para Educação Infantil que são alinhados à BNCC!18 maio 2024 -
 VicTsing 2.4G Wireless Gaming Mouse, USB Cordless PC Computer Mice with Silent Click, Auto-sleep Mode, 7 Buttons, 5 Adjustable DPI, Plug & Play Wireless Mouse for Game PC Laptop Computer Mac18 maio 2024
VicTsing 2.4G Wireless Gaming Mouse, USB Cordless PC Computer Mice with Silent Click, Auto-sleep Mode, 7 Buttons, 5 Adjustable DPI, Plug & Play Wireless Mouse for Game PC Laptop Computer Mac18 maio 2024 -
 Digimon Adventure (2020 TV series) - Wikiwand18 maio 2024
Digimon Adventure (2020 TV series) - Wikiwand18 maio 2024 -
 🌟 CHEAP SALE 🌟 Roblox - Grand Piece Online - GPO - Devil Fruits18 maio 2024
🌟 CHEAP SALE 🌟 Roblox - Grand Piece Online - GPO - Devil Fruits18 maio 2024 -
 FNAF The Joy of Creation - All Jumpscares + Gameplay demo v0.618 maio 2024
FNAF The Joy of Creation - All Jumpscares + Gameplay demo v0.618 maio 2024